hand out for coagulation tests with sysmex5100
COAGULATION CASCADE
blood clotting mechanism is explained by cascade theory.
it consists of 2 major division
primary coagulation
secondary coagulation.
Primary coagulation.
Primary coagulation, also known as primary hemostasis, is the initial phase of blood clotting that occurs in response to vascular injury. It involves the formation of a temporary platelet plug to stop bleeding. When blood vessels are damaged, platelets adhere to the site of injury, become activated, and aggregate to form a plug. This process is mediated by various factors including von Willebrand factor (vWF), collagen exposed by injury, and the release of platelet agonists such as ADP and thromboxane A2. Once the platelet plug is formed, it provides a surface for the activation of the coagulation cascade, leading to the formation of a stable blood clot through secondary hemostasis. Primary coagulation is essential for the prevention of excessive bleeding and the initiation of the overall hemostatic process.
further it can be explained as
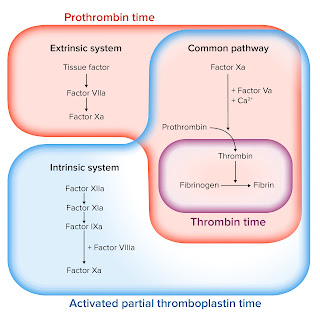
Intrinsic, extrinsic, and common pathways
intrinsic pathway
The intrinsic pathway is one of the two main pathways involved in the coagulation cascade, which is the series of events that leads to the formation of a blood clot. The intrinsic pathway is initiated within the bloodstream itself and is activated by factors present within the blood.
Key steps in the intrinsic pathway include:
Activation: The intrinsic pathway is initiated when blood comes into contact with a negatively charged surface, such as collagen exposed in the subendothelial matrix of damaged blood vessels. This interaction activates factor XII (also known as Hageman factor).
Factor XII activation: Upon activation, factor XII converts to its active form, factor XIIa, which then activates factor XI (also known as plasma thromboplastin antecedent or PTA).
Factor XI activation: Factor XIa, in turn, activates factor IX (also known as Christmas factor).
Factor IX activation: Factor IXa, along with its cofactor factor VIII (also known as antihemophilic factor A), forms a complex on a phospholipid surface that activates factor X (also known as Stuart-Prower factor).
Factor X activation: Factor Xa is generated, leading to the activation of the common pathway of coagulation, which ultimately results in the conversion of prothrombin to thrombin.
Thrombin then converts fibrinogen into fibrin strands, which polymerize and cross-link to form a stable blood clot.
The intrinsic pathway works in conjunction with the extrinsic pathway, which is initiated by tissue factor (TF) released from damaged tissues outside of the bloodstream. Both pathways converge at the activation of factor X, leading to the formation of thrombin and the subsequent clotting cascade.
The intrinsic pathway is important for amplifying the coagulation response and ensuring efficient clot formation in response to vascular injury.
extrinsic pathway
The extrinsic pathway is one of the two main pathways involved in the coagulation cascade, which is the series of events that leads to the formation of a blood clot. The extrinsic pathway is initiated by tissue factor (TF), also known as factor III, which is released from damaged tissues outside of the bloodstream.
Key steps in the extrinsic pathway include:
1. Tissue injury: When tissues are injured, tissue factor (TF) is exposed to the bloodstream.
2. TF activation: TF binds to and activates factor VII (also known as proconvertin) that circulates in the blood. This binding converts factor VII to its active form, factor VIIa.
3. Factor VII activation: Factor VIIa, in conjunction with tissue factor, forms a complex that activates factor X (also known as Stuart-Prower factor).
4. Factor X activation: Factor Xa is generated, which then activates the common pathway of coagulation, ultimately leading to the conversion of prothrombin to thrombin.
5. Thrombin formation: Thrombin converts fibrinogen into fibrin strands, which polymerize and cross-link to form a stable blood clot.
The extrinsic pathway works in conjunction with the intrinsic pathway, which is initiated within the bloodstream itself by factors present in the blood. Both pathways converge at the activation of factor X, leading to the formation of thrombin and subsequent clotting cascade.
The extrinsic pathway is considered the primary initiator of the coagulation cascade in response to tissue injury and plays a crucial role in initiating the clotting process rapidly to prevent excessive bleeding.
common pathway
The common pathway of the coagulation cascade is the final stage where both the intrinsic and extrinsic pathways converge. This convergence leads to the activation of a series of clotting factors that ultimately culminate in the formation of a stable blood clot. The main events of the common pathway include:
1. Activation of Factor X: Factor X, which has been activated either through the extrinsic pathway (by factor VIIa and tissue factor) or the intrinsic pathway (by the intrinsic pathway factors VIIIa, IXa, XIa, and phospholipids), is further activated into Factor Xa.
2. Prothrombin Activation: Factor Xa combines with factor Va (a cofactor) and phospholipids on the platelet surface to form the prothrombinase complex. This complex then converts prothrombin (Factor II) into thrombin (Factor IIa), a key enzyme in the coagulation cascade.
3. Thrombin Formation: Thrombin plays a central role in blood clot formation. It converts soluble fibrinogen into insoluble fibrin strands. Fibrin strands form a meshwork that traps blood cells, creating a stable blood clot.
4. Fibrin Polymerization and Cross-Linking: Fibrin monomers polymerize and cross-link with each other, forming a mesh-like structure that reinforces the platelet plug, creating a stable blood clot.
5. Clot Maturation and Consolidation: The stable blood clot undergoes maturation and consolidation processes, leading to its contraction and the retraction of the clot, which helps in wound healing and tissue repair.
The common pathway is crucial for amplifying the initial signals from both the intrinsic and extrinsic pathways, leading to the rapid and efficient formation of a blood clot in response to vascular injury. It represents the final steps in the coagulation cascade, ultimately leading to the cessation of bleeding and the initiation of tissue repair processes.
secondary hemostasis
Secondary hemostasis, also known as the coagulation cascade, is the second stage in the process of hemostasis, which is the body's mechanism for stopping bleeding. It occurs following primary hemostasis, which involves the formation of a platelet plug at the site of vascular injury.
The primary purpose of secondary hemostasis is to reinforce the platelet plug with a stable blood clot. This is achieved through a series of complex biochemical reactions involving various clotting factors, which are present in the blood plasma. The process occurs in three main stages:
1. **Initiation:** Secondary hemostasis can be triggered by tissue factor (extrinsic pathway) or by exposure of blood to collagen (intrinsic pathway). Tissue factor interacts with factor VII, leading to the activation of factor X. In the intrinsic pathway, collagen exposure activates factor XII, which subsequently activates factor XI, leading to the activation of factor IX. These pathways ultimately converge to activate factor X.
2. **Amplification:** Activated factor X (Xa) combines with factor V (Va) and calcium ions on the platelet surface to form the prothrombinase complex. This complex converts prothrombin (factor II) into thrombin (factor IIa). Thrombin is a key enzyme in the coagulation cascade, and it further activates additional factors in the cascade, amplifying the clotting response.
3. **Propagation:** Thrombin not only converts fibrinogen into fibrin but also activates factor XIII, which cross-links fibrin strands to form a stable fibrin meshwork. This meshwork reinforces the platelet plug, creating a stable blood clot.
Secondary hemostasis results in the formation of a fibrin clot that strengthens and stabilizes the initial platelet plug, effectively sealing the wound and preventing further bleeding. It is a tightly regulated process, with various inhibitors and feedback mechanisms to ensure that clot formation occurs only at the site of vascular injury and does not lead to excessive clotting elsewhere in the body.
tests measure intrinsic pathway
The activated partial thromboplastin time (aPTT) test is commonly used to measure the function of the intrinsic pathway of the coagulation cascade. Here's how it works:
1. **Blood Collection**: A blood sample is collected from the patient via venipuncture using a needle and syringe or vacutainer tube.
2. **Preparation of Plasma**: The blood sample is typically collected into a tube containing an anticoagulant, such as citrate, to prevent clotting. The tube is then centrifuged to separate the plasma from the cellular components of the blood.
3. **Reagent Addition**: To initiate the intrinsic pathway, a specific reagent, typically a partial thromboplastin reagent, is added to the plasma sample. This reagent contains phospholipids and an activator such as kaolin or silica, which help to activate the intrinsic pathway factors.
4. **Calcium Addition**: Calcium chloride or another calcium source is added to the plasma sample to enable the coagulation cascade to proceed.
5. **Clot Formation Measurement**: The time it takes for a fibrin clot to form in the plasma sample is measured. This is typically done using an automated coagulation analyzer, which monitors changes in optical density or clot formation kinetics.
6. **Interpretation**: The aPTT result is reported in seconds and reflects the time taken for the intrinsic pathway to form a fibrin clot. Prolongation of the aPTT may indicate deficiencies or abnormalities in one or more of the intrinsic pathway clotting factors, such as factor VIII, IX, XI, or XII.
The aPTT test is used clinically to evaluate patients with suspected bleeding disorders, to monitor anticoagulant therapy (such as heparin), and to assess the function of the intrinsic pathway in coagulation.
Tests for extrinsic pathway
The prothrombin time (PT) test is commonly used to measure the function of the extrinsic pathway of the coagulation cascade. Here's how it works:
1. **Blood Collection**: A blood sample is collected from the patient via venipuncture using a needle and syringe or vacutainer tube.
2. **Preparation of Plasma**: The blood sample is typically collected into a tube containing an anticoagulant, such as citrate, to prevent clotting. The tube is then centrifuged to separate the plasma from the cellular components of the blood.
3. **Reagent Addition**: To initiate the extrinsic pathway, a specific reagent called tissue factor (TF) is added to the plasma sample. TF is a protein that is normally found outside the bloodstream but is added to the test to activate the extrinsic pathway.
4. **Calcium Addition**: Calcium chloride or another calcium source is added to the plasma sample to enable the coagulation cascade to proceed.
5. **Clot Formation Measurement**: The time it takes for a fibrin clot to form in the plasma sample is measured. This is typically done using an automated coagulation analyzer, which monitors changes in optical density or clot formation kinetics.
6. **Interpretation**: The PT result is reported in seconds and reflects the time taken for the extrinsic pathway to form a fibrin clot. Prolongation of the PT may indicate deficiencies or abnormalities in one or more of the extrinsic pathway clotting factors, such as factor VII.
The PT test is used clinically to evaluate patients with suspected bleeding disorders, to monitor anticoagulant therapy (such as warfarin), and to assess the function of the extrinsic pathway in coagulation.
common pathway tests
To assess the function of the common pathway of the coagulation cascade, there are several laboratory tests that can be performed. These tests provide information on the overall coagulation process and the integrity of the common pathway. Here are some of the common tests used:
1. **Prothrombin Time (PT)**: The PT test measures the time it takes for blood to clot after the addition of tissue factor (a substance that triggers the extrinsic pathway) and calcium. It primarily evaluates the function of factors in the extrinsic and common pathways, including factors I (fibrinogen), II (prothrombin), V, VII, and X. Prolongation of PT can indicate deficiencies or abnormalities in these factors.
2. **Activated Partial Thromboplastin Time (aPTT)**: The aPTT test measures the time it takes for blood to clot after the addition of an activator (such as kaolin or ellagic acid) and calcium. It primarily evaluates the function of factors in the intrinsic and common pathways, including factors I (fibrinogen), II (prothrombin), V, VIII, IX, X, XI, and XII. Prolongation of aPTT can indicate deficiencies or abnormalities in these factors.
3. **Thrombin Time (TT)**: The TT test measures the time it takes for fibrinogen to be converted to fibrin in the presence of thrombin. It evaluates the final step of the coagulation cascade, which involves the conversion of fibrinogen to fibrin. Prolongation of TT can indicate abnormalities in fibrinogen levels or function.
4. **Fibrinogen Assay**: This test directly measures the concentration of fibrinogen in the blood. Fibrinogen is a protein essential for blood clot formation, and abnormal levels can affect clotting function.
5. **D-Dimer Assay**: Elevated levels of D-dimer, a breakdown product of fibrin clot degradation, can indicate the presence of thrombus formation or fibrinolysis (clot breakdown). This test is often used to diagnose conditions such as deep vein thrombosis (DVT), pulmonary embolism (PE), and disseminated intravascular coagulation (DIC).
These tests are routinely used in clinical practice to evaluate patients with suspected bleeding disorders, clotting disorders, or thrombotic events. They provide valuable information about the function of the coagulation cascade and help guide diagnosis and treatment decisions.
Comments
Post a Comment